FDA emite uso de emergencia para la vacuna contra covid19 de Pfizer
Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original And Omicron BA.4/BA.5) AUTHORIZED USE. Pfizer-BioNTech COVID-19 Vaccine, Bivalent (Original and Omicron BA.4/BA.5) is FDA-authorized underEmergency Use Authorization (EUA) for use in individuals 12 years of age and older as a single booster dose administered at least 2 months after either:
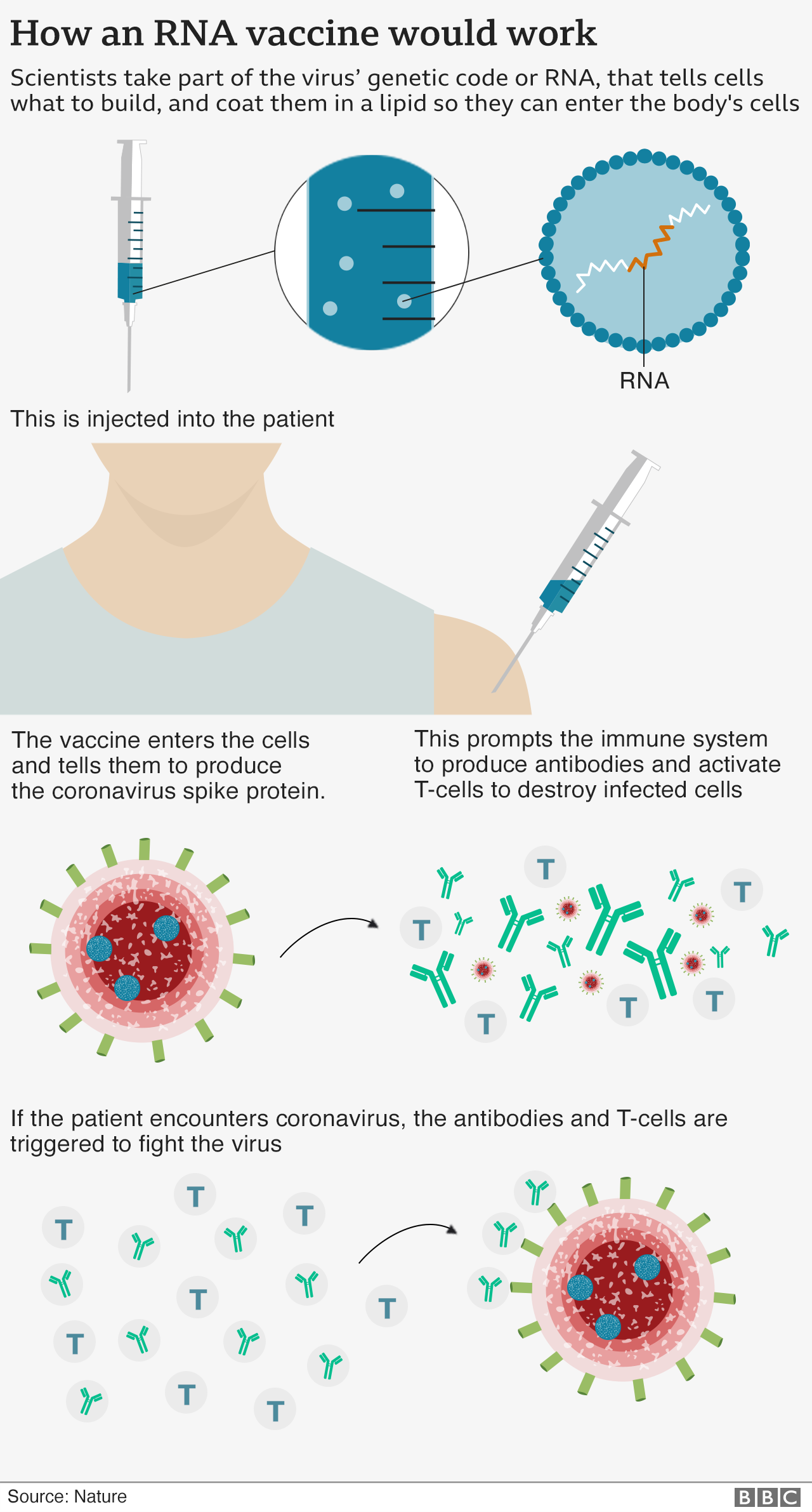
Covid vaccine Pfizer says '94 effective in over65s' BBC News
ATAGI advises that the Pfizer bivalent BA.4/5 vaccine can be used as a booster dose by adolescents and adults aged ≥12 years who are recommended to receive a COVID-19 booster according to the ATAGI 2023 booster advice. The Pfizer bivalent BA.4/5 vaccine is not currently registered for use in children aged <12 years or as a primary series .

As Moderna looks to increase the doses in vaccine vials, the White House announces an expected
Health Canada recently approved an updated Pfizer-BioNTech Comirnaty COVID-19 vaccine targeting the dominant circulating Omicron subvariants BA.4 and BA.5, as well as the original strain of the virus.

COVID bivalent booster dose Moderna or Pfizer? When should I get it?
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that the U.S. Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for a 10-µg booster dose of their Omicron BA.4/BA.5-adapted bivalent COVID-19 vaccine in children 5 through 11 years of age. Pending recommendation from the Centers for Disease Control and Prevention (CDC), 10-µg doses will be.
Vaccine Ready
Here, we report data from a large cohort study on the effectiveness of these two bivalent vaccines against severe infection with omicron BA.4.6, BA.5, BQ.1, and BQ.1.1.
Pfizer to begin vaccine production in Dublin BBC News
Objective To estimate the effectiveness of the bivalent mRNA booster vaccines containing the original SARS-CoV-2 and omicron BA.4-5 or BA.1 subvariants as the fourth dose against severe covid-19. Design Nationwide cohort analyses, using target trial emulation. Setting Denmark, Finland, Norway, and Sweden, from 1 July 2022 to 10 April 2023. Participants People aged ≥50 years who had received.
Moderna vaccine safe and effective, say US experts BBC News
Today, Health Canada authorized the Pfizer-BioNTech Comirnaty Omicron BA.4/BA.5 bivalent-adapted booster for use in children 5 to 11 years of age. This is the first bivalent COVID-19 booster authorized for use in this age group that targets the original COVID-19 strain and the Omicron BA.4/BA.5 subvariants.

Does the Bivalent Booster Entirely Replace Other Covid19 Boosters? The New York Times
The research cohorts included 3,368,697 adults vaccinated with the fourth dose, of which 1,290,999 were bivalent BA.4-5-vaccinated, 992,282 were bivalent BA.1-vaccinated, and 1,085,416 were.

COVID Pfizer, Moderna omicron bivalent boosters confuse providers
In a small study, a bivalent mRNA vaccine targeting omicron BA.4-BA.5 sublineages resulted in similar neutralization of other SARS-CoV-2 variants as a fourth dose of a monovalent mRNA vaccine.
Pfizer's Covid19 vaccine study excluded people with a history of severe allergic reactions
Bivalent booster elicited approximately 4-fold higher neutralizing antibody titers against Omicron BA.4/BA.5 sublineages compared to the original COVID-19 vaccine in individuals older than 55 years of age One-month after a 30-µg booster dose of the bivalent vaccine, Omicron BA.4/BA.5-neutralizing antibody titers increased 13.2-fold from pre-booster levels in adults older than 55 years of age.

First data on PfizerBioNTech's Omicron BA.4/5 combination vaccine
Omicron BA.4/5, and bivalent versions of the ARVAC vaccine, used as a booster in adult. R.LP., C.G.M., LP, V.M.TU, C.J.W, and R.Z. conduct clinical trials for Pfizer, Merck, Sanofi, and Moderna, Inc. P.B. was investigator in clinical trials of Sinopharm and Janssen COVID-19 vaccines and was Advisory Board Participant for Moderna and Raffo..

Mixing Pfizer, AstraZeneca Vaccines Gives Strong Covid Protection, Study Finds The New York Times
Pfizer's new BA.4/5 bivalent vaccine differs from its previous formulation by replacing mRNA of the BA.1 Omicron subvariant with mRNA that encodes the BA.4/5 spike protein instead.
Quan chức Trung Quốc nói vaccine nội địa 'không có tỷ lệ bảo vệ cao' BBC News Tiếng Việt
For Product Information and Consumer Medicine Information about Comirnaty bivalent Original/Omicron BA.4/5 visit the Therapeutic Goods Administration website. Additional vaccine information For detailed advice on vaccine dosage, administration, contraindications and precautions, and variations from product information, please visit the relevant.
Ecuador will receive 4 million doses of PfizerBioNTech vaccine
The Pfizer-BioNTech Bivalent COVID-19 Vaccine does not contain preservative, and the vial stoppers are not made with naratu l rubber latex. NDC 59267-0304-1: Multiple dose vial; contains six doses of 0.3 mL. BIVALENT (Original and Omicron BA.4/BA.5) [ GRAY cap with GRAY Label Border] Formulation, Storage, Preparation, Dispensing and.

SPIKEVAX Bivalent (Original / Omicron BA.4/5) (elasomeran/davesomeran) COVID19 Vaccine with
This was the first research project conducted to evaluate the immunity induced by the Pfizer-BioNTech bivalent vaccine (COMIRNATY Original/omicron BA.4-5) in a group of Brazilian subjects.
Latest COVID Bivalent Booster Now Available at QNHCH The Queen′s Health System
On Wednesday 20 December 2023, the Therapeutic Goods Administration (TGA) granted provisional approval to Pfizer's bivalent COVID-19 vaccine: tozinameran and famtozinameran (COMIRNATY Original/Omicron BA.4-5 COVID-19 vaccine) for use as a booster dose in individuals aged 5 years and older. The new bivalent vaccine comprises 5 micrograms of famtozinameran based on the Omicron variants BA.4.